computer
Computer
modelling of a corrosion cell and the effects of cathodic protection.
A 'MODEL' CORROSION CELL.
It is possible to make a computer model of a corrosion cell, based on the laws of electricity, on any spread sheet such as Lotus 123 or Microsoft Excel for Windows.
|
|
|
|
|
|
|
|
|
|
|
|
amps |
|
|
|
|
Metal Ohms |
0 |
|
0.3 |
0 |
Metal Ohms |
|
|
Metal potential |
0 |
|
|
0 |
Metal potential |
|
|
Cathode EMF |
0 |
|
|
1.2 |
Anode EMF |
|
|
Interface Ohms |
2 |
|
|
1.2 |
Electrolyte potential |
|
|
Electrolyte potential |
0.6 |
|
0.3 |
2 |
Close electrolyte Ohms |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Corrosion cell (a) |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Each cell is used to represent a finite area of the metal or the electrolyte and is given a value in ohms, amps or volts. The values of cells representing the resistance in Ohms are operator variable but all the others calculate their own value from the adjustment of the 'reaction EMF' shown in volts.
The operator sets the values of the resistances, and by adjusting the EMF can see the amount of current flowing and the potentials that result from the voltage drop around the corrosion circuit.
Each cell in the model is related to other cells which would influence it. The calculations necessary to make this model work are all formulae based on Ohms Law, and the building of this model is simple but informative.
CATHODIC PROTECTION MODEL
It is possible to superimpose a model of an impressed current cathodic protection system over this model of a corrosion cell. This can be made to arrest the reaction of the corrosion reaction. In this way it is possible to see the adjustment of the transformer rectifier reducing the corrosion current to nil.
This concept can be extended to many cells of differing reactions and with different resistances in their corrosion circuits. The effect of a single cathodic protection system can be superimposed over many different cells and the complexities of reality can be imagined from the mass of calculations necessary to make this limited, simple, two dimensional model work.
Of course, the basic model is in two dimensions and reality is in three dimensions which would make modelling more complex, and the number of calculations greater, by an order of magnitude.
It might seem that it is impossible to model a real cathodic protection system, for many of the variables cannot be defined and the amount of variables might be regarded as infinite.
However, there is sufficient confirmed data to which the computer can be applied, to achieve much better results than possible manually.
The computer also makes it possible to pass on concepts of cathodic protection.
The notion of a groundbed can be put into values and help build a mental picture of the charges dispersing through the earth to the pipe metal.
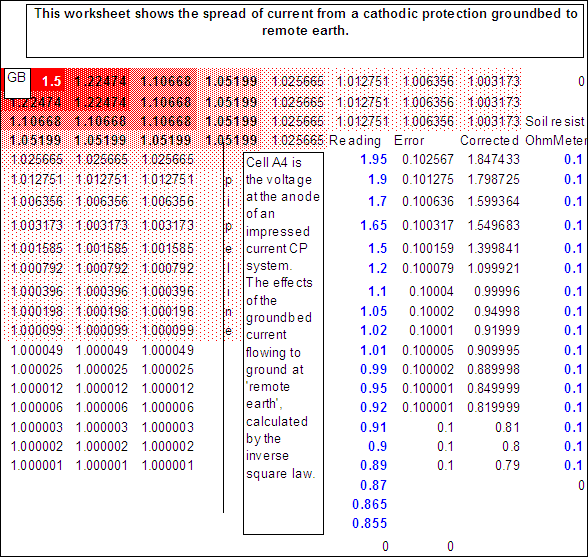
This notion can be confirmed by measuring voltages at ground level over a groundbed against a fixed voltage.
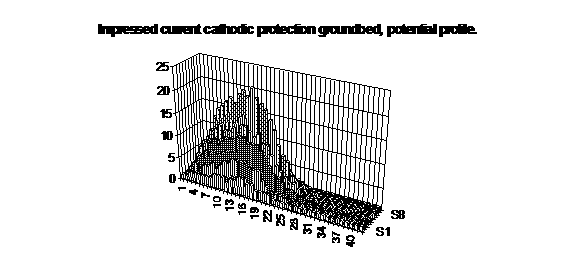
The computer can actually calculate the corrected values of the measured voltages to refer to a common reference potential.
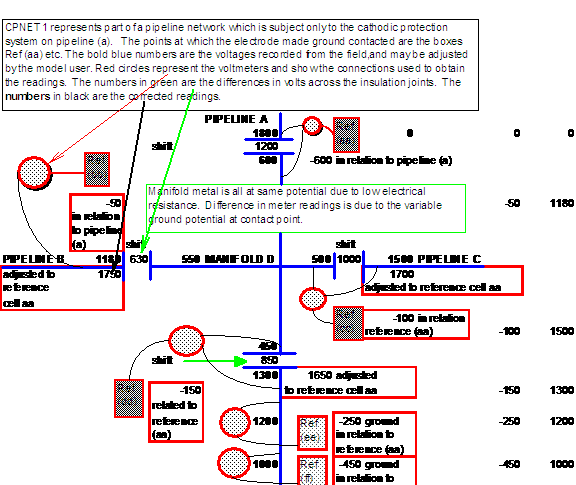
This will, for the first time, give a picture of the real potentials of the earth in relation to the whole pipeline network and will indicate the paths of stray currents, which can cause accellerated corrosion.
It will be possible to adjust the settings on the model transformer-rectifiers and observe the effect on the whole system without the expense of field surveys to monitor the results.
In short, this project would allow a corrosion engineer to view the whole of his cathodic protection and attain a true balance which will ensure complete protection.